Intern special
Learn ” Sampling ”
———————–
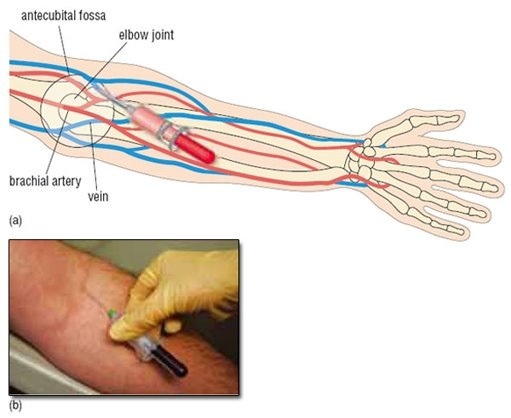
Venous Blood Sampling
************************
1- Apply a tourniquet proximal to the
venepuncture site.
2- Tap on or rub the skin overlying the
vein you want to puncture lightly to
make it standout; or if the vein is
obscure, you may be able to palpate its
course with you index finger.
3- Sterilization with alcohol and leave to
dry in air
(traces of alcohol causes hemolysis)
4- Hold the syringe between the thumb
and the middle, ring and small finger of
your dominant hand and steady the
needle using your index finger
.
” Never hold the syringe like a pencil because it limits your angle of skin
entry ”
5- confirme entry of the vein by
aspiration of blood, Once the desired
volume of blood is aspirated into the
syringe, steady it with one hand and
release the tourniquet with the other
hand.
6- Use a piece of dry gauze to apply
gentle pressure to the entry site while
the needle is withdrawn. Ask the
patient to continue to apply pressure
while you attend to the specimen
obtained .
7- Remove the needle from the syringe
and drop it into the special box for
disposal of sharp instruments
immediately .
8- Uncap the specimen container and
deposit the blood sample into it directly
from the syringe. Injecting blood into
the container via the needle is not
advisable because:
(a) you may prick
yourself and
(b) forcing blood through the needle may cause hemolysis that
can adversely affect the analytical
fitness of the specimen.
■ Avoid hemolysis:-
Allow alcohol to dry
Clean tubes
Withdraw blood slowly
Do not use too fine needle
Deliver blood gently to the tube Avoid frothing
Share or tag ur self…..!
Category Archives: Endocrinology
Best info about HYPO & HYPER THYROIDSM
Hyperthyroidism is characterized by hypermetabolism and elevated serum levels of free thyroid hormones. Symptoms are many but include tachycardia, fatigue, weight loss, nervousness, and tremor. Diagnosis is clinical and with thyroid function tests. Treatment depends on cause.
Hyperthyroidism can be classified on the basis of thyroid radioactive iodine uptake and the presence or absence of circulating thyroid stimulators (see Thyroid Disorders: Results of Thyroid Function Tests in Various Clinical Situations).
Etiology
Hyperthyroidism may result from increased synthesis and secretion of thyroid hormones (thyroxine [T4] and triiodothyronine [T3]) from the thyroid, caused by thyroid stimulators in the blood or by autonomous thyroid hyperfunction. It can also result from excessive release of thyroid hormone from the thyroid without increased synthesis. Such release is commonly caused by the destructive changes of various types of thyroiditis. Various clinical syndromes also cause hyperthyroidism.
Graves’ disease (toxic diffuse goiter), the most common cause of hyperthyroidism, is characterized by hyperthyroidism and one or more of the following:
Goiter
Exophthalmos
Infiltrative dermopathy
Graves’ disease is caused by an autoantibody against the thyroid receptor for thyroid-stimulating hormone (TSH); unlike most autoantibodies, which are inhibitory, this autoantibody is stimulatory, thus causing continuous synthesis and secretion of excess T4 and T3. Graves’ disease (like Hashimoto’s thyroiditis) sometimes occurs with other autoimmune disorders, including type 1 diabetes mellitus, vitiligo, premature graying of hair, pernicious anemia, connective tissue diseases, and polyglandular deficiency syndrome. The pathogenesis of infiltrative ophthalmopathy (responsible for the exophthalmos in Graves’ disease) is poorly understood but may result from immunoglobulins directed to specific receptors in the orbital fibroblasts and fat that result in release of proinflammatory cytokines, inflammation, and accumulation of glycosaminoglycans. Ophthalmopathy may also occur before the onset of hyperthyroidism or as late as 20 yr afterward and frequently worsens or abates independently of the clinical course of hyperthyroidism. Typical ophthalmopathy in the presence of normal thyroid function is called euthyroid Graves’ disease.
Inappropriate TSH secretion is a rare cause. Patients with hyperthyroidism have essentially undetectable TSH except for those with a TSH-secreting anterior pituitary adenoma or pituitary resistance to thyroid hormone. TSH levels are high, and the TSH produced in both disorders is biologically more active than normal TSH. An increase in the α-subunit of TSH in the blood (helpful in differential diagnosis) occurs in patients with a TSH-secreting pituitary adenoma.
Molar pregnancy, choriocarcinoma, and hyperemesis gravidarum produce high levels of serum human chorionic gonadotropin (hCG), a weak thyroid stimulator. Levels of hCG are highest during the 1st trimester of pregnancy and result in the decrease in serum TSH and mild increase in serum free T4 sometimes observed at that time. The increased thyroid stimulation may be caused by increased levels of partially desialated hCG, an hCG variant that appears to be a more potent thyroid stimulator than more sialated hCG. Hyperthyroidism in molar pregnancy, choriocarcinoma, and hyperemesis gravidarum is transient; normal thyroid function resumes when the molar pregnancy is evacuated, the choriocarcinoma is appropriately treated, or the hyperemesis gravidarum abates.
Nonautoimmune autosomal dominant hyperthyroidism manifests during infancy. It results from mutations in the TSH receptor gene that produce continuous thyroid stimulation.
Toxic solitary or multinodular goiter (Plummer’s disease) sometimes results from TSH receptor gene mutations producing continuous thyroid stimulation. Patients with toxic nodular goiter have none of the autoimmune manifestations or circulating antibodies observed in patients with Graves’ disease. Also, in contrast to Graves’ disease, toxic solitary and multinodular goiters usually do not remit.
Inflammatory thyroid disease (thyroiditis) includes subacute granulomatous thyroiditis, Hashimoto’s thyroiditis, and silent lymphocytic thyroiditis, a variant of Hashimoto’s thyroiditis (see Thyroid Disorders: Silent Lymphocytic Thyroiditis). Hyperthyroidism results from destructive changes in the gland and release of stored hormone, not from increased synthesis. Hypothyroidism may follow.
Drug-induced hyperthyroidism can result from amiodarone and interferon alfa, which may induce thyroiditis with hyperthyroidism and other thyroid disorders. Although more commonly causing hypothyroidism, lithium can rarely cause hyperthyroidism. Patients receiving these drugs should be closely monitored.
Thyrotoxicosis factitia is hyperthyroidism resulting from conscious or accidental overingestion of thyroid hormone.
Excess iodine ingestion causes hyperthyroidism with a low thyroid radioactive iodine uptake. It most often occurs in patients with underlying nontoxic nodular goiter (especially elderly patients) who are given drugs that contain iodine (eg, amiodarone, iodine-containing expectorants) or who undergo radiologic studies using iodine-rich contrast agents. The etiology may be that the excess iodine provides substrate for functionally autonomous (ie, not under TSH regulation) areas of the thyroid to produce hormone. Hyperthyroidism usually persists as long as excess iodine remains in the circulation.
Metastatic thyroid cancer is a possible cause. Overproduction of thyroid hormone occurs rarely from functioning metastatic follicular carcinoma, especially in pulmonary metastases.
Struma ovarii develops when ovarian teratomas contain enough thyroid tissue to cause true hyperthyroidism. Radioactive iodine uptake occurs in the pelvis, and uptake by the thyroid is usually suppressed.
Pathophysiology
In hyperthyroidism, serum T3 usually increases more than does T4, probably because of increased secretion of T3 as well as conversion of T4 to T3 in peripheral tissues. In some patients, only T3 is elevated (T3 toxicosis). T3 toxicosis may occur in any of the usual disorders that cause hyperthyroidism, including Graves’ disease, multinodular goiter, and the autonomously functioning solitary thyroid nodule. If T3 toxicosis is untreated, the patient usually also develops laboratory abnormalities typical of hyperthyroidism (ie, elevated T4 and 123I uptake). The various forms of thyroiditis commonly have a hyperthyroid phase followed by a hypothyroid phase.
Symptoms and Signs
Most symptoms and signs are the same regardless of the cause. Exceptions include infiltrative ophthalmopathy and dermopathy, which occur only in Graves’ disease.
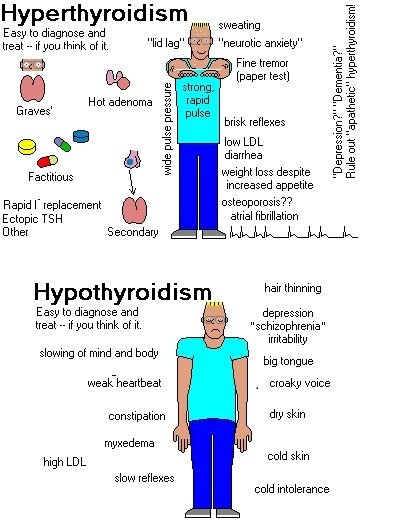
The clinical presentation may be dramatic or subtle. A goiter or nodule may be present. Many common symptoms and signs of hyperthyroidism are similar to those of adrenergic excess, such as nervousness, palpitations, hyperactivity, increased sweating, heat hypersensitivity, fatigue, increased appetite, weight loss, insomnia, weakness, and frequent bowel movements (occasionally diarrhea). Hypomenorrhea may be present. Signs may include warm, moist skin; tremor; tachycardia; widened pulse pressure; atrial fibrillation; and palpitations.
Elderly patients, particularly those with toxic nodular goiter, may present atypically (apathetic or masked hyperthyroidism) with symptoms more akin to depression or dementia. Most do not have exophthalmos or tremor. Atrial fibrillation, syncope, altered sensorium, heart failure, and weakness are more likely. Symptoms and signs may involve only a single organ system.
Eye signs include stare, eyelid lag, eyelid retraction, and mild conjunctival injection and are largely due to excessive adrenergic stimulation. They usually remit with successful treatment. Infiltrative ophthalmopathy, a more serious development, is specific to Graves’ disease and can occur years before or after hyperthyroidism. It is characterized by orbital pain, lacrimation, irritation, photophobia, increased retro-orbital tissue, exophthalmos, and lymphocytic infiltration of the extraocular muscles, causing ocular muscle weakness that frequently leads to double vision.
Diagnosis
TSH
Free T4
Sometimes radioactive iodine uptake
Diagnosis is based on history, physical examination, and thyroid function tests. Serum TSH measurement is the best test, because TSH is suppressed in hyperthyroid patients except in the rare instance when the etiology is a TSH-secreting pituitary adenoma or pituitary resistance to thyroid hormone. Screening selected populations for TSH level is warranted (see Thyroid Disorders: Laboratory Testing of Thyroid Function). Free T4 is increased in hyperthyroidism. However, T4 can be falsely normal in true hyperthyroidism in patients with a severe systemic illness (similar to the falsely low levels that occur in euthyroid sick syndrome) and in T3 toxicosis. If free T4 level is normal and TSH is low in a patient with subtle symptoms and signs of hyperthyroidism, then serum T3 should be measured to detect T3 toxicosis; an elevated level confirms that diagnosis.
The cause can often be diagnosed clinically (eg, exposure to a drug, the presence of signs specific to Graves’ disease). If not, thyroid radioactive iodine uptake may be obtained by using 123I. When hyperthyroidism is due to hormone overproduction, thyroid radioactive iodine uptake is usually elevated.
TSH receptor antibodies can be measured to detect Graves’ disease, but measurement is rarely necessary except during the 3rd trimester of pregnancy to assess the risk of neonatal Graves’ disease; TSH receptor antibodies readily cross the placenta to stimulate the fetal thyroid. Most patients with Graves’ disease have circulating antithyroid peroxidase antibodies, and fewer have antithyroglobulin antibodies.
Inappropriate TSH secretion is uncommon. The diagnosis is confirmed when hyperthyroidism occurs with elevated circulating free T4 and T3 concentrations and normal or elevated serum TSH.
If thyrotoxicosis factitia is suspected, serum thyroglobulin can be measured; it is usually low or low-normal—unlike in all other causes of hyperthyroidism.
In hyperthyroidism caused by excess iodine ingestion, low radioactive iodine uptake is typical because thyroid radioactive iodine uptake is inversely proportional to iodine intake.
Treatment
Treatment depends on cause but may include
Propylthiouracil or methimazole
β-Blockers
Iodine
Radioactive iodine
Surgery
Iodine
Iodine in pharmacologic doses inhibits the release of T3 and T4 within hours and inhibits the organification of iodine, a transitory effect lasting from a few days to a week, after which inhibition usually ceases. Iodine is used for emergency management of thyroid storm, for hyperthyroid patients undergoing emergency nonthyroid surgery, and (because it also decreases the vascularity of the thyroid) for preoperative preparation of hyperthyroid patients undergoing subtotal thyroidectomy. Iodine generally is not used for routine treatment of hyperthyroidism. The usual dosage is 2 to 3 drops (100 to 150 mg) of a saturated K iodide solution po tid or qid or 0.5 to 1 g Na iodide in 1 L 0.9% saline solution given IV slowly q 12 h.
Complications of iodine therapy include inflammation of the salivary glands, conjunctivitis, and rash.
Propylthiouracil and methimazole
These antithyroid drugs block thyroid peroxidase, decreasing the organification of iodide, and impair the coupling reaction. Propylthiouracil in high doses also inhibits the peripheral conversion of T4 to T3. About 20 to 50% of patients with Graves’ disease remain in remission after a 1- to 2-yr course of either drug. The return to normal or a marked decrease in gland size, the restoration of a normal serum TSH level, and less severe hyperthyroidism before therapy are good prognostic signs of long-term remission. The concomitant use of antithyroid drug therapy and l-thyroxine does not improve the remission rate in patients with Graves’ disease. Because toxic nodular goiter rarely goes into remission, antithyroid drug therapy is given only in preparation for surgical treatment or 131I therapy.
The usual starting dosage of propylthiouracil is 100 to 150 mg po q 8 h and of methimazole 5 to 20 mg po tid. When T4 and T3 levels normalize, the dosage is decreased to the lowest effective amount, usually propylthiouracil 50 mg tid or methimazole 5 to 15 mg once/day. Usually, control is achieved in 2 to 3 mo. More rapid control can be achieved by increasing the dosage of propylthiouracil to 150 to 200 mg q 8 h. Such dosages or higher ones (up to 400 mg q 8 h) are generally reserved for severely ill patients, including those with thyroid storm. Maintenance doses can be continued for one or many years depending on the clinical circumstances. Carbimazole, which is used widely in Europe, is rapidly converted to methimazole. The usual starting dose is similar to that of methimazole; maintenance dosage is 5 to 20 mg po once/day, 2.5 to 10 mg bid, or 1.7 to 6.7 mg tid.
Adverse effects include rash, allergic reactions, abnormal liver function, and, in about 0.1% of patients, reversible agranulocytosis. Patients allergic to one drug can be switched to the other, but cross-sensitivity may occur. If agranulocytosis occurs, the patient cannot be switched to the other drug; other therapy (eg, radioiodine, surgery) should be used.
Each drug has advantages and disadvantages. Methimazole need only be given once/day, which improves adherence. Furthermore, when methimazole is used in dosages of 65. Although typically easy to diagnose in younger adults, it may be subtle and manifest atypically in the elderly.
Primary hypothyroidism
Primary hypothyroidism is due to disease in the thyroid; thyroid-stimulating hormone (TSH) is increased. The most common cause is probably autoimmune. It usually results from Hashimoto’s thyroiditis and is often associated with a firm goiter or, later in the disease process, with a shrunken fibrotic thyroid with little or no function. The 2nd most common cause is post-therapeutic hypothyroidism, especially after radioactive iodine therapy or surgery for hyperthyroidism or goiter. Hypothyroidism during overtreatment with propylthiouracil, methimazole, and iodide abates after therapy is stopped.
Most patients with non-Hashimoto’s goiters are euthyroid or have hyperthyroidism, but goitrous hypothyroidism may occur in endemic goiter. Iodine deficiency decreases thyroid hormonogenesis. In response, TSH is released, which causes the thyroid to enlarge and trap iodine avidly; thus, goiter results. If iodine deficiency is severe, the patient becomes hypothyroid, a rare occurrence in the US since the advent of iodized salt.
Iodine deficiency can cause endemic cretinism in children; endemic cretinism is the most common cause of congenital hypothyroidism in severely iodine-deficient regions and a major cause of mental deficiency worldwide.
Rare inherited enzymatic defects can alter the synthesis of thyroid hormone and cause goitrous hypothyroidism (see Endocrine Disorders in Children: Congenital Goiter).
Hypothyroidism may occur in patients taking lithium, perhaps because lithium inhibits hormone release by the thyroid. Hypothyroidism may also occur in patients taking amiodarone or other iodine-containing drugs, and in patients taking interferon alfa. Hypothyroidism can result from radiation therapy for cancer of the larynx or Hodgkin lymphoma (Hodgkin’s disease). The incidence of permanent hypothyroidism after radiation therapy is high, and thyroid function (through measurement of serum TSH) should be evaluated at 6- to 12-mo intervals.
Secondary hypothyroidism
Secondary hypothyroidism occurs when the hypothalamus produces insufficient thyrotropin-releasing hormone (TRH) or the pituitary produces insufficient TSH. Sometimes, deficient TSH secretion due to deficient TRH secretion is termed tertiary hypothyroidism.
Symptoms and Signs
Symptoms and signs of primary hypothyroidism are often subtle and insidious. Symptoms may include cold intolerance, constipation, forgetfulness, and personality changes. Modest weight gain is largely the result of fluid retention and decreased metabolism. Paresthesias of the hands and feet are common, often due to carpal-tarsal tunnel syndrome caused by deposition of proteinaceous ground substance in the ligaments around the wrist and ankle. Women with hypothyroidism may develop menorrhagia or secondary amenorrhea.
The facial expression is dull; the voice is hoarse and speech is slow; facial puffiness and periorbital swelling occur due to infiltration with the mucopolysaccharides hyaluronic acid and chondroitin sulfate; eyelids droop because of decreased adrenergic drive; hair is sparse, coarse, and dry; and the skin is coarse, dry, scaly, and thick. The relaxation phase of deep tendon reflexes is slowed. Hypothermia is common. Dementia or frank psychosis (myxedema madness) may occur.
Carotenemia is common, particularly notable on the palms and soles, caused by deposition of carotene in the lipid-rich epidermal layers Deposition of proteinaceous ground substance in the tongue may cause macroglossia. A decrease in both thyroid hormone and adrenergic stimulation causes bradycardia. The heart may be enlarged, partly because of dilation but chiefly because of pericardial effusion. Pleural or abdominal effusions also may be noted. The pericardial and pleural effusions develop slowly and only rarely cause respiratory or hemodynamic distress.
Elderly patients have significantly fewer symptoms than do younger adults, and complaints are often subtle and vague. Many elderly patients with hypothyroidism present with nonspecific geriatric syndromes—confusion, anorexia, weight loss, falling, incontinence, and decreased mobility. Musculoskeletal symptoms (especially arthralgias) occur often, but arthritis is rare. Muscular aches and weakness, often mimicking polymyalgia rheumatica or polymyositis, and an elevated CK level may occur. In the elderly, hypothyroidism may mimic dementia or parkinsonism.
Although secondary hypothyroidism is uncommon, its causes often affect other endocrine organs controlled by the hypothalamic-pituitary axis. In a woman with hypothyroidism, indications of secondary hypothyroidism are a history of amenorrhea rather than menorrhagia and some suggestive differences on physical examination. Secondary hypothyroidism is characterized by skin and hair that are dry but not very coarse, skin depigmentation, only minimal macroglossia, atrophic breasts, and low BP. Also, the heart is small, and serous pericardial effusions do not occur. Hypoglycemia is common because of concomitant adrenal insufficiency or growth hormone deficiency.
Myxedema coma: Myxedema coma is a life-threatening complication of hypothyroidism, usually occurring in patients with a long history of hypothyroidism. Its characteristics include coma with extreme hypothermia (temperature 24° to 32.2° C), areflexia, seizures, and respiratory depression with CO2 retention. Severe hypothermia may be missed unless low-reading thermometers are used. Rapid diagnosis based on clinical judgment, history, and physical examination is imperative, because death is likely without rapid treatment. Precipitating factors include illness, infection, trauma, drugs that suppress the CNS, and exposure to cold.
Diagnosis
TSH
Free thyroxine (T4)
Serum TSH is the most sensitive test, and screening of selected populations is warranted (see Thyroid Disorders: Laboratory Testing of Thyroid Function). In primary hypothyroidism, there is no feedback inhibition of the intact pituitary, and serum TSH is always elevated, whereas serum free T4 is low. In secondary hypothyroidism, free T4 and serum TSH are low (sometimes TSH is normal but with decreased bioactivity).
Many patients with primary hypothyroidism have normal circulating levels of triiodothyronine (T3), probably caused by sustained TSH stimulation of the failing thyroid, resulting in preferential synthesis and secretion of biologically active T3. Therefore, serum T3 is not sensitive for hypothyroidism.
Anemia is often present, usually normocytic-normochromic and of unknown etiology, but it may be hypochromic because of menorrhagia and sometimes macrocytic because of associated pernicious anemia or decreased absorption of folate. Anemia is rarely severe (Hb > 9 g/dL). As the hypometabolic state is corrected, anemia subsides, sometimes requiring 6 to 9 mo.
Serum cholesterol is usually high in primary hypothyroidism but less so in secondary hypothyroidism.
In addition to primary and secondary hypothyroidism, other conditions may cause decreased levels of total T4, such as serum thyroxine-binding globulin (TBG) deficiency, some drugs (see Thyroid Disorders: Primary hypothyroidism), and euthyroid sick syndrome (see Thyroid Disorders: Hashimoto’s Thyroiditis).
Treatment
l-Thyroxine, adjusted until TSH levels are in midnormal range
Various thyroid hormone preparations are available for replacement therapy, including synthetic preparations of T4 (l-thyroxine), T3 (liothyronine), combinations of the 2 synthetic hormones, and desiccated animal thyroid extract. L-Thyroxine is preferred; the usual maintenance dose is 75 to 150 μg po once/day, depending on age, body mass index, and absorption (for pediatric doses, see Endocrine Disorders in Children: Treatment). Therapy is begun with low doses, especially in the elderly, usually 25 μg once/day. The dose is adjusted every 6 wk until maintenance dose is achieved. The maintenance dose may need to be decreased in elderly patients and increased in pregnant women. Dose may also need to be increased if drugs that decrease T4 absorption or increase its biliary excretion are administered concomitantly. The dose used should be the lowest that restores serum TSH levels to the midnormal range (though this criterion cannot be used in patients with secondary hypothyroidism).
Liothyronine should not be used alone for long-term replacement because of its short half-life and the large peaks in serum T3 levels it produces. The administration of standard replacement amounts (25 to 37.5 μg bid) results in rapidly increasing serum T3 to between 300 and 1000 ng/dL (4.62 to 15.4 nmol/L) within 4 h due to its almost complete absorption; these levels return to normal by 24 h. Additionally, patients receiving liothyronine are chemically hyperthyroid for at least several hours a day, potentially increasing cardiac risks.
Similar patterns of serum T3 occur when mixtures of T3 and T4 are taken po, although peak T3 is lower because less T3 is given. Replacement regimens with synthetic T4 preparations reflect a different pattern in serum T3 response. Increases in serum T3 occur gradually, and normal levels are maintained when adequate doses of T4 are given. Desiccated animal thyroid preparations contain variable amounts of T3 and T4 and should not be prescribed unless the patient is already taking the preparation and has normal serum TSH.
In patients with secondary hypothyroidism, l-thyroxine should not be given until there is evidence of adequate cortisol secretion (or cortisol therapy is given), because l-thyroxine could precipitate adrenal crisis.
Myxedema coma
Myxedema coma is treated as follows:
T4 given IV
Corticosteroids
Supportive care as needed
Conversion to oral T4 when patient is stable
Patients require a large initial dose of T4 (300 to 500 μg IV) or T3 (25 to 50 μg IV). The IV maintenance dose of T4 is 75 to 100 μg once/day and of T3, 10 to 20 μg bid until T4 can be given orally. Corticosteroids are also given, because the possibility of central hypothyroidism usually cannot be initially ruled out. The patient should not be rewarmed rapidly, which may precipitate hypotension or arrhythmias. Hypoxemia is common, so Pao2 should be monitored. If ventilation is compromised, immediate mechanical ventilatory assistance is required. The precipitating factor should be rapidly and appropriately treated and fluid replacement given carefully, because hypothyroid patients do not excrete water appropriately. Finally, all drugs should be given cautiously because they are metabolized more slowly than in healthy people.
Subclinical Hypothyroidism
Subclinical hypothyroidism is elevated serum TSH in patients with absent or minimal symptoms of hypothyroidism and normal serum levels of free T4.
Subclinical thyroid dysfunction is relatively common; it occurs in more than 15% of elderly women and 10% of elderly men, particularly in those with underlying Hashimoto’s thyroiditis.
In patients with serum TSH > 10 mU/L, there is a high likelihood of progression to overt hypothyroidism with low serum levels of free T4 in the next 10 yr. These patients are also more likely to have hypercholesterolemia and atherosclerosis. They should be treated with l-thyroxine, even if they are asymptomatic. For patients with TSH levels between 4.5 and 10 mU/L, a trial of l-thyroxine is reasonable if symptoms of early hypothyroidism (eg, fatigue, depression) are present. l-Thyroxine therapy is also indicated in pregnant women and in women who plan to become pregnant to avoid deleterious effects of hypothyroidism on the pregnancy and fetal development. Patients should have annual measurement of serum TSH and free T4 to assess progress of the condition if untreated or to adjust the l-thyroxine dosage.